The PAH synthesis at UiS began as a bachelor thesis driven project to provide analytical reference materials for environmental scientists at our local environmental research facilities, now NORCE.
Since 2002, more than 35 bachelor students have been involved in photochemical synthesis of chrysenes and phenanthrenes.
After my sabbatical at Victor Snieckus lab in Queen’s University in Canada in 2008 we also began using his DoM/DreM methodology to make phenanthrenes, lately also expanded to 6- and 7-ring PAHs made from photochemically provided chrysenes. Science becomes easier when PhD-students are available. The need of environmental scientists is still a driving force, but research and collaborations developed through the COST-action CHAOS has also inspired a move towards larger molecules and material science, provided success in grant proposals.
Here are some results from my labs:
DoM/DreM strategies:
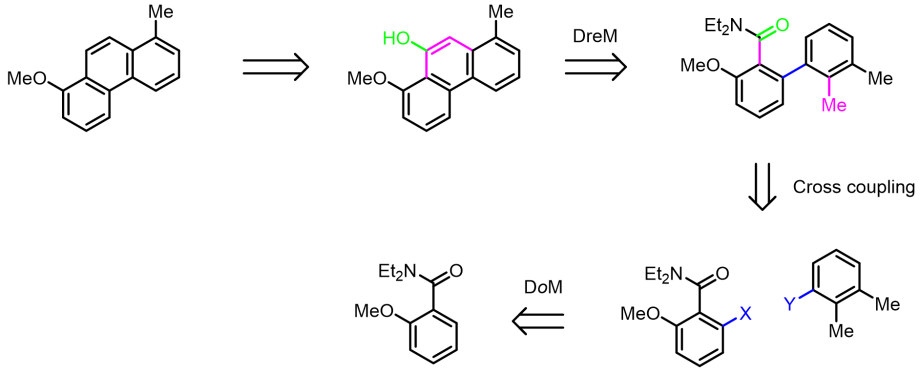
There are several directing groups (DMGs) that can be used to direct a Lithium base at low temperatures to deprotonate a certain hydrogen in a molecule, partly overriding the internal acidity. This is a versatile way to introduce substituents ortho to the DMG, like halogen and boronic acids to set up a Suzuki-Miyaura cross-coupling. In a biaryl at room temperature Lithium bases can also be directed by the DMG to metalate a methyl group in a remote location.
With an amide group as DMG the result becomes a cyclization forming a phenanthrene from a biphenyl as indicated above.
We have used this methodology to introduce the methoxy-group (later removed to make phenols as metabolite standards of methylated PAHs) in every position of 1-methylphenanthrene. The method is also useful for making dimethylated PAHs as single isomers.
Ref:
J. Org Chem. 80 (19), 9410-9424 (2015)
Polycyclic aromatic compounds, 37(2-3), 106-113 (2017)
The method also allows the construction of larger PAHs:
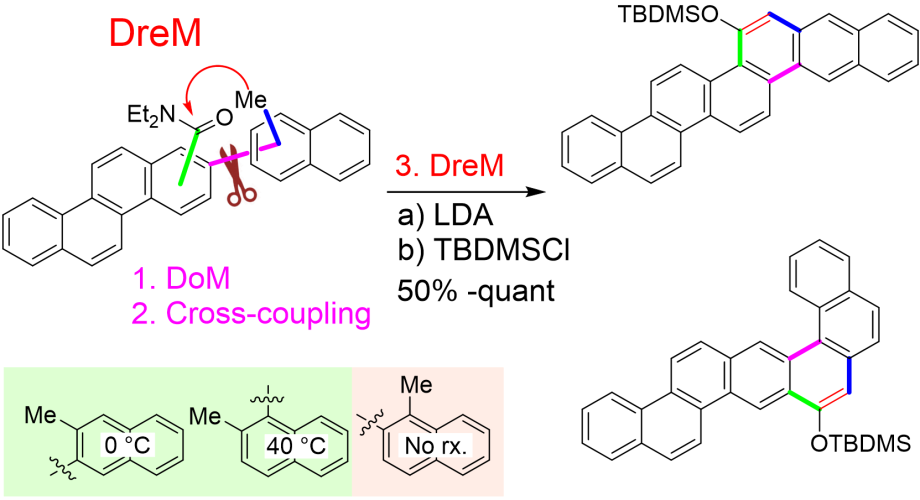
Ref:
J. Org. Chem., under review.
Although these biaryls become quite crowded and display some atropisomerism on the NMR timescale, even the most crowded biaryl make a combined rotation of the DMG and the other aryl to allow rotation at a reduced rotational barrier that should allow formation of the DreM-intermediate:

Ref:
J. Org. Chem. 82(14), 7300-7308 (2017)
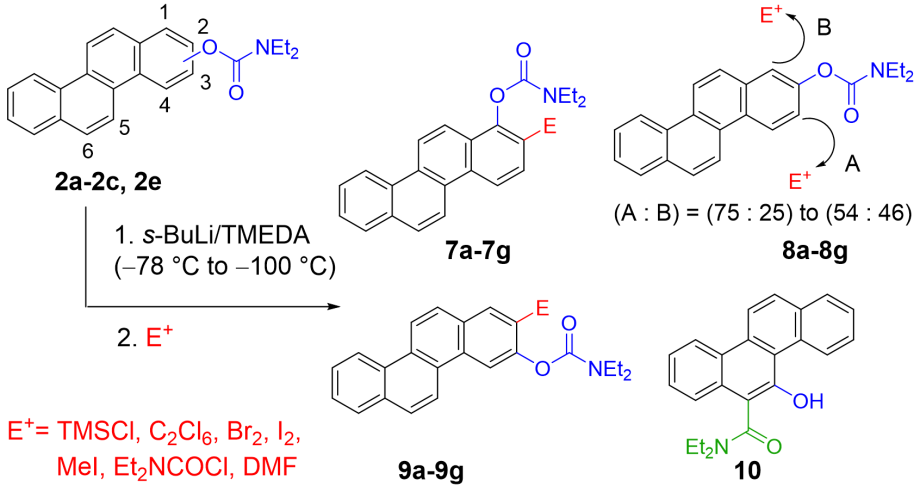
Directed ortho metalation on chrysenes has also been investigated. Again, we see differences from results on benzene or naphthalene.
The steric bulk of the molecule blocks the bay-position and allow regiospecific substitution in 2-postion from 3-carbamates, while this DMG undergoes instant Fries-rearrangement from the 5-position upon metalation (forming 10):
Current group members
Department of Chemistry, Bioscience and Environmental Engineering
Department of Chemistry, Bioscience and Environmental Engineering

